views
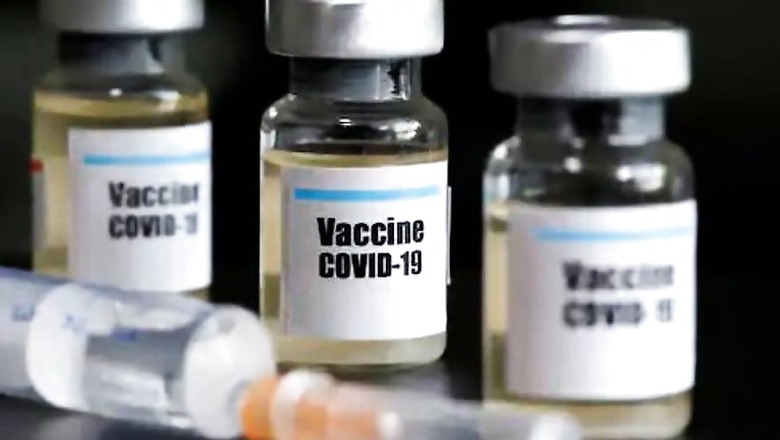
Russian President Vladimir Putin surprised the world on Tuesday as he announced the launch of 'Sputnik V', the government’s home-grown vaccine against Covid-19.
Named after the Sputnik, the world’s first satellite launched into space by the Soviet Union, Putin claimed that the vaccine was "quite effective", gave sustainable immunity and added for good measure that one of his daughters had already been inoculated.
However, little else was revealed about the vaccine, which has been developed by the Gamaleya Research National Research Institute of Epidemiology and Microbiology, and has been funded by Russian Direct Investment Fund.
So what is it this vaccine, how does it function? How many trials has it been subjected to? News18 answers all questions in this explainer:
What is the Sputnik Vaccine?
Sputnik V is a human adenovirus vector-based vaccine made from a weakened version of a common cold virus - the adenovirus. A somewhat similar virus platform has been used by scientists at the Oxford University, who have used a weakened version of the common cold virus that causes infections in chimpanzees.
The viral vector platform relies on a virus that lacks a gene which is responsible for reproduction, and is used to deliver the genetic material from another virus being vaccinated against, into a cell. The vector used as a delivery platform does not pose harm and triggers an immune reaction in the vaccinated person.
The Gamaleya Institute, which has developed the vaccine, uses two vectors unlike the Oxford vaccine. These two adenovirus vectors carry the gene coding S protein of the SARS-CoV-2 spikes which have now become famous in representative images of the novel coronavirus. “The use of two vectors is a unique technology of the Gamaleya Center making the Russian vaccine different from other adenovirus vector-based vaccines being developed globally,” a Russian government website on the vaccine said.
How Safe is the Vaccine & How Many Trials Has it Been Subjected to?
The Sputnik V vaccine has been subject to pre-clinical trials involving two types of primates as per the vaccine project’s official website. Pre-clinical trials are meant to test safety, toxicity and efficacy among animals. According to the vaccine's project website, Phase 1 and Phase 2 clinical trials of the vaccine were completed on August 1, 2020. Phase 3 clinical trials involving more than 2,000 people in Russia and others in UAE, Saudia Arabia, Brazil and Mexico will start from August 12.
None of the clinical trials results have been published in public domain or in a peer reviewed journal for the scientific community to review the findings. In contrast, the Oxford University vaccine group published preliminary results of the vaccine’s safety and efficacy in a peer-reviewed journal.
The Sputnik vaccine group claimed that all volunteers are feeling well, and that no unforeseen or unwanted side effects were observed. “The vaccine induced strong antibody and cellular immune response. Not a single participant of the current clinical trials got infected with Covid-19 after being administered with the vaccine,” the vaccine group’s website claimed.
What Were the Parameters and Primary Outcomes Set for the Trials?
The Phase 1 and Phase 2 studies looked to evaluate the safety, tolerability, immunogenicity of the vaccine. The trials were open, two stage non-randomised in Phase 2 with participation of health volunteers. Only 38 participants were enrolled for the trials. The primary outcome that the trial aimed to look at was a change in antibody levels against coronavirus and adverse events in the participants.
What Other Vaccines Has Gamayela Research Institute Worked on?
The Russian vaccine group website said that the Gamaleya Center had been working on adenoviral vector-based vaccines since the 1980s. The Gamaleya Center in the past had developed an adenoviral vector-based vaccine against Ebola. However, the World Health Organisation still terms it as an experimental vaccine.
“Another adenoviral vector-based vaccine against Middle East Respiratory Syndrome (MERS) is in advanced stages of clinical trials,” the website of the vaccine group said.
“During the vaccine creation process a gene with the code of a protein from a spike of coronavirus is inserted into an adenoviral vector. This inserted element is safe for the body but still helps the immune system to react and produce antibodies, which protect us from the infection,” they added.

















Comments
0 comment