views
Making Homemade pH Paper with Cabbage
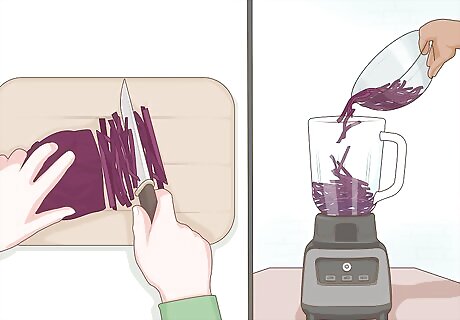
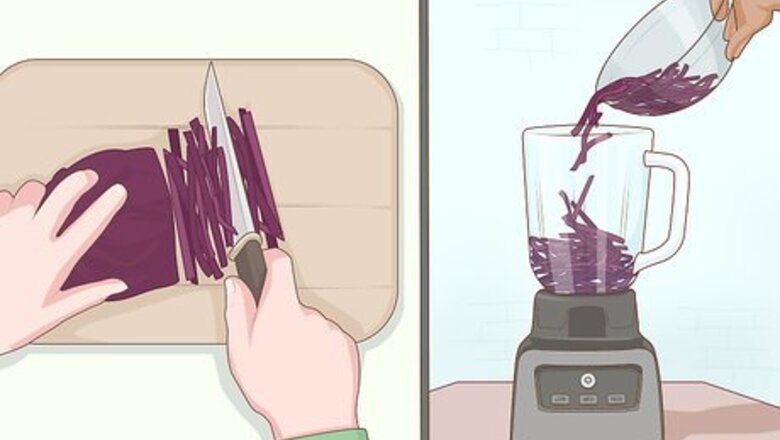
Chop up some red cabbage. You'll need to chop about ¼ of a head of red cabbage and put it in a blender. You will extract chemicals from the cabbage to coat your pH paper. These chemicals are known as anthocyanins and are found in plants such as cabbage, roses, and berries. Anthocyanins are purple under neutral conditions (pH 7.0) but they change color when exposed to an acid (pH < 7.0) or a base (pH>7.0). The same procedure can be followed using berries, roses, and other anthocyanin containing plants. This does not work for green cabbage. The same anthocyanins are not present in green cabbage.
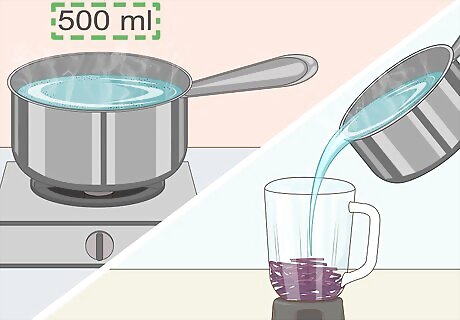
Add boiling water to your cabbage. You can boil the water on a stovetop or in the microwave, but either way you'll need about 500 mL of water. Pour the boiling water directly into the blender with the cabbage. This will help draw the needed chemicals out of the cabbage.

Turn on the blender. You need to blend the water and cabbage for best results. Keep the mixture blending until the water is dark purple. This color change indicates that you have successfully drawn the needed chemicals (anthocyanins) from the cabbage and dissolved them in the hot water. You should allow the contents of the blender to cool for at least ten minutes before proceeding.
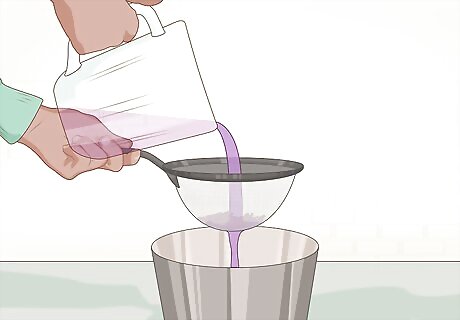
Pour the mix through a strainer. You want to remove any pieces of cabbage from the indicator solution (colored water). Filter paper will work in place of a strainer, but may take more time. Once you have strained the indicator solution, you can throw away the cabbage pieces.

Add isopropyl alcohol to your indicator solution. Adding about 50 mL of isopropyl alcohol will protect your solution from bacterial growth. The alcohol may start to alter the color of your solution. If this happens, add vinegar until the solution goes back to dark purple. You can substitute ethanol for isopropyl alcohol, if necessary or desired.

Pour the solution into a pan or bowl. You want a container with a wide enough opening to dip your paper. You should choose a container that is stain-resistant, as you are pouring dyes into it. Ceramic and glass are good options.

Soak your paper in the indicator solution. Make sure to push the paper all the way to the bottom. You want to cover all corners and edges of the paper. It is a good idea to use gloves for this step.
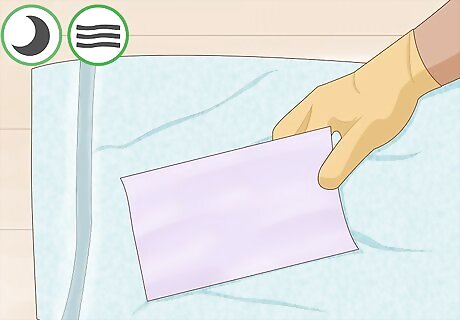
Allow your paper to air dry on a towel. Find a location that is free of acidic or basic vapors. The paper should be allowed to dry completely before proceeding. Ideally, you will leave it overnight.
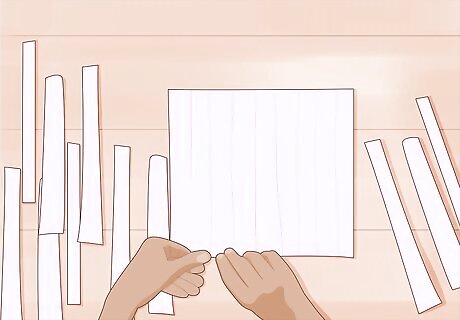
Cut the paper into strips. This will allow you to test several different samples. You can cut the strips any size you would like, but generally the length and width of your index finger is fine. This will allow you to dip the strip into a sample without getting your fingers into the sample.

Use the strips to test the pH of different solutions. You can test household solutions such as orange juice, water, and milk. You can also mix up solutions for testing, e.g. mixing water and baking soda. This will give you a wide range of samples to test.
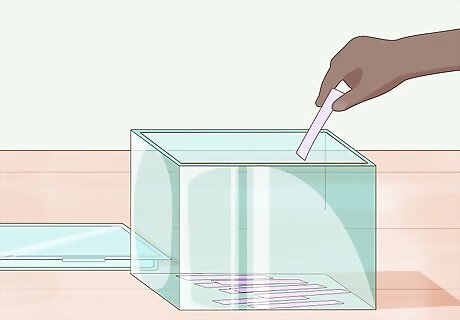
Store the strips in cool dry place. You should use an airtight container to store the strips until you use them. This will protect them from environmental contamination such as acidic or basic gases. It is also ideal not to leave them in direct sunlight, as this could result in bleaching over time.
Making Homemade Litmus Paper

Obtain dry litmus powder. Litmus is a compound that is derived from lichens, fungi that form symbiotic relationships with alga and/or cyanobacteria capable of photosynthesis. You can purchase litmus powder online or at a chemical supply store. It is possible to make your own litmus powder if you are a competent chemist. However, the process is quite involved and includes adding several compounds such as lime and potash to ground lichens and allowing weeks for fermentation.

Dissolve the litmus into water. Make sure to stir the solution and heat if the powder is not dissolving well. The litmus powder needs to dissolve completely into the water. The resulting solution should be a violet-blue color.

Submerge white acid-free art paper in the litmus solution. Get all sides and corners of the paper wet with the solution. This will give you the most surface area on the test strip and provide the most accurate results. You do not need to leave the paper to “soak” as long as you ensure that it is coated thoroughly.
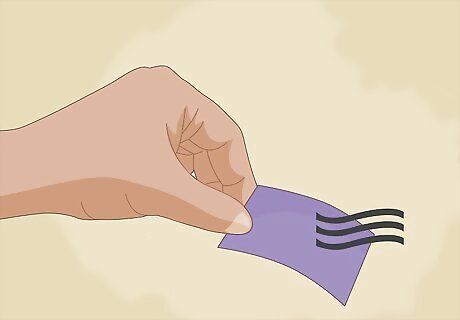
Allow your paper to dry. You should dry the paper in the open air, but be sure that you are not exposing it to acidic or basic vapors. These vapors could contaminate the strips and make the inaccurate. You should also be sure to store them in a dry, dark place to prevent contamination and bleaching.

Use the litmus paper to test for acidity. Blue litmus papers turn red in the presence of an acid. Keep in mind that they will not indicate how strong the acid is, or whether a solution is basic. No change means that the solution is either basic or neutral, but not acidic. You can make red litmus paper (that turns blue when exposed to a base) by adding acid to the indicator solution before soaking your paper.


















Comments
0 comment