views
X
Trustworthy Source
U.S. Geological Survey
U.S. government agency responsible for conducting scientific research on the nation's land, natural resources, and natural disasters
Go to source
Finding the Values of Your Variables

Measure the mass of your equipment before starting. If you are calculating densities of liquids or gasses especially, you will need to know the mass of the container. This way, you can subtract the mass from the total mass when you measure the object’s mass. Place your empty beaker, jar, or other container onto a scale, and write down its mass in grams. Some scales allow you to “tare” the weight. This means once you have the container on the scale, you will press “tare”, and the scale will set the new mass equal to zero. This subtracts out the mass of anything on the scale for you.
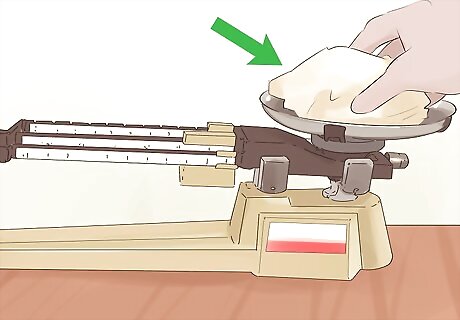
Place your object on the scale to find its mass. Either by itself, if it’s a solid, or in its container, for liquids and gasses, measure the mass using your scale. Record the mass in grams, subtracting the mass of any container you have used.

Convert the mass to grams if it is in another unit. Some scales will measure in units other than grams. If yours will not measure in grams, you may need to convert the units by multiplying the value by a conversion value. 1 ounce is approximately equal to 28.35 grams.4 1 pound is about 453.59 grams. In these cases, you will multiply the mass of your object by the conversion factor of 28.35 to convert ounces to grams or 453.59 for pounds to grams.
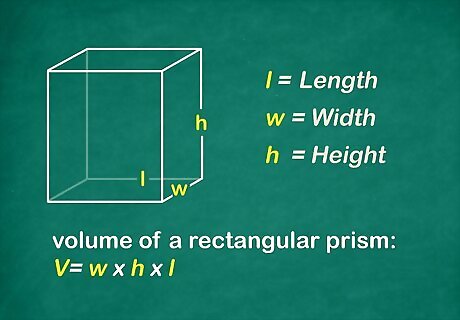
Find the volume of the object in cubic centimeters. If you are fortunate enough to have a rectangular solid, you can simply measure the length, width, and height of the object in centimeters. You will then multiply the 3 digits together for the volume.
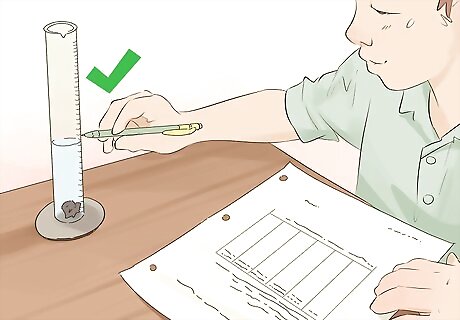
Determine the volume for non-rectangular solids. For liquids or gasses, you will need to use a graduated cylinder or beaker to record the volume. For solids in other odd shapes, you will either need to use the right equation or submerge it in water to calculate the volume. 1 milliliter is equal to 1 cubic centimeter. This makes liquid and gas conversions quite simple! There are different mathematical formulas for finding the volume of a rectangle, a cylinder, a pyramid, and so on. If the object is solid and non-porous with no regular dimensions to measure, such as a jagged rock, you can calculate its volume by immersing it in water and measuring the volume of water it displaces. By Archimedes' principle, an object displaces a volume of liquid equal to its own volume. Simply subtract the original volume of the liquid from the combined volume of liquid with the solid in it.
Using the Density Equation
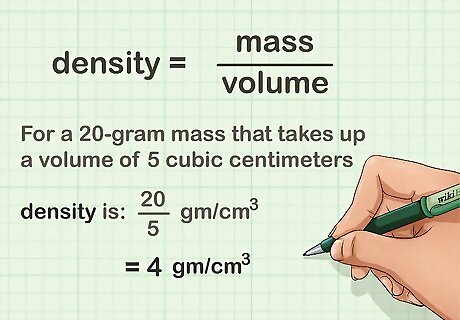
Divide the object's mass by its volume. Using a calculator, or by hand, if you are feeling adventurous, simply divide the mass in grams by the volume in cubic centimeters. For a 20-gram mass that takes up a volume of 5 cubic centimeters, the density is 4 grams per cubic centimeter.

Simplify your answer to the appropriate number of significant digits. Real-world values are not typically as clean cut as you might find in word problems. Therefore, when you divide real mass values by volumes, you are likely to get a long number with many decimal places. Consult your teacher or whomever the calculation is for to ask how many significant figures are needed. Usually rounding to 2 or 3 digits past the decimal place is plenty accurate. Therefore, a number like 32.714907 could be rounded down to 32.71 or 32.715 g/cm.

Evaluate the meaning of the density. Typically density of an object is related to the density of water (1.0 g/cm). If your object’s density is greater than 1.0, it will sink in water. Otherwise, it will float. The same relationship will be true for other liquids. For example, when you try to mix olive oil and water, the oil will rise to the top since it is less dense than water. Specific gravity is another ratio that relates to density. Often it is the density of an object divided by the density of water (or another substance). The units cancel each other out, so you are just left with a number that represents relative masses. The number is often used in chemistry to determine concentrations of a substance in a solution.



















Comments
0 comment