views
As coronavirus batters countries around the world, Pfizer CEO Albert Bourla said that an oral drug used for treating the deadly infection could be ready by next year.
Bourla explained that the company is testing two antivirals, one that is injected intravenously and another that is administered orally.
Talking to CNBC in an interview on Tuesday, Albert Bourla said that though the company is working on two products, its focus remains on the oral drug as it can be administered at home, unlike the injection for which one has to visit the hospital.
Shedding light on the oral drug, the CEO said that this form of medication may come to be more effective against the multiple variants of the virus than the current ones adding that the action of the antiviral, is not expected to be subject to mutations.
“This one doesn’t work on the spike so that allows us to believe that will be way more effective against the multiple variants. So, all good news. We are now progressing the studies and we will have more news around summer,” the CEO was quoted saying to CNBC.
When asked what will be the reasonable time frame before the drug comes in the market, he added the company plans to provide a detailed update over the summer.
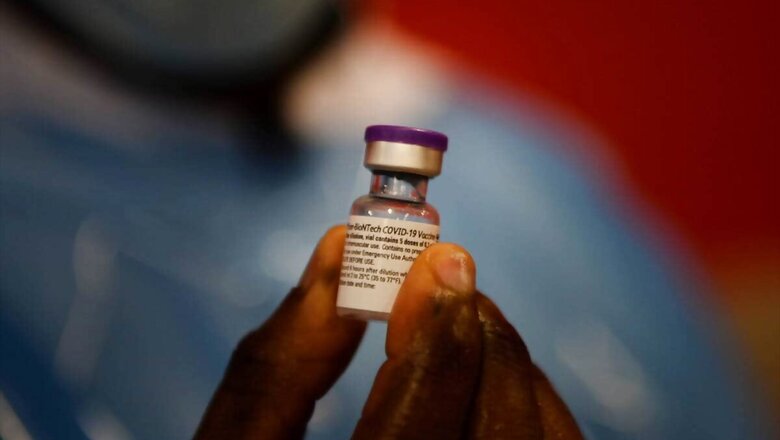
Pfizer will produce at least 2.5 billion doses of their vaccine this year, which equates to 3 billion doses on an annual basis, the CEO said.
With 3,359,963 more Covid-19 vaccine doses being administered on Monday, India’s total count of vaccine shots so far reached 145,271,186. The count of recovered Covid-19 cases across India, meanwhile, reached 14,556,209 of total caseload with 251,827 new cured cases being reported on Tuesday.Pfizer
Read all the Latest News, Breaking News and Coronavirus News here. Follow us on Facebook, Twitter and Telegram.

















Comments
0 comment