views
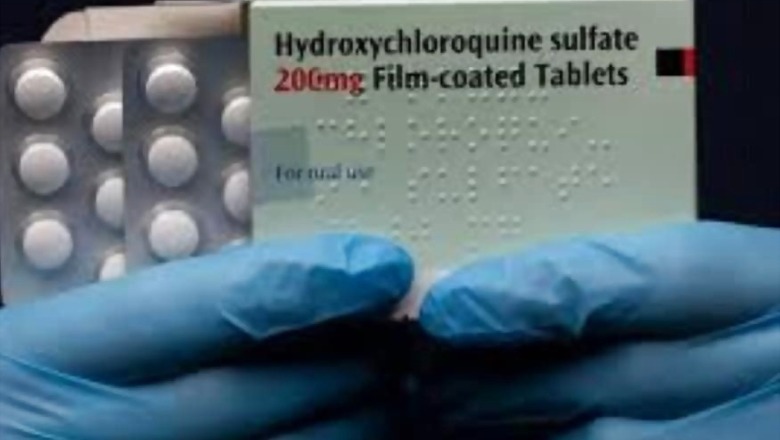
Amid rising concerns on hydroxychloroquine and the World Health Organisation’s (WHO) decision to press pause on the HCQ arm of global solidarity trial, India will continue with its use as preventive care against COVID-19 among healthcare workers.
Dr Balram Bhargava, the Director General of Indian Council of Medical Research (ICMR), said the drug was recommended initially in March based on in-vitro studies which showed it had antiviral properties.
Meanwhile, Bhargava said, there have been observational studies and case control studies that have not shown many side-effects due to the use of HCQ.
“We thought it might be useful drug for prevention of COVID-19 and taking the biological plausibility, the in-vitro data, and taking the availability and safety of this drug, we recommended it as empiric use under strict medical supervision,” Bhargava said during the central government’s briefing on COVID-19.
“During the weeks since it was recommended, we got some data (on HCQ) in India and showed that there is no harm, but benefit may be there,” Bhargava said.
“They were mainly observational studies in different cohorts done at AIIMS and case control study at ICMR and studies were also done at three Delhi public hospitals. We found that it may be working and there were no major side effects, except nausea, vomiting and some palpitations occasionally. We have clearly said it should be continued for prophylaxis,” Bhargava said.
The ICMR DG said the government’s latest advisory regarding expanding the use of HCQ to frontline workers such as paramilitary and police personnel is based on “risk-benefit analysis”.
“We should not deny this to our healthcare workers and frontline workers who are dealing with virus-hit patients. At the same time, we have also said that PPE use must be continued. Our study on HCQ will be published soon,” he said.
ICMR had first recommended use of the anti-malarial drug in March and expanded its use last week.
However, almost at the same time, a large observational study published in The Lancet done on patients in six continents concluded the drug could also have a harmful affect by way of increasing risk of irregular heartbeat.
WHO Director General Tedros Adhanom Ghebreysus said on Monday the UN body’s executive group had implemented a temporary pause of the HCQ arm within the solidarity trial while the data is reviewed by the data-safety monitoring.
"The review will consider data collected so far in the Solidarity Trial and, in particular, robust randomised available data, to adequately evaluate the potential benefits and harms from this drug,” the WHO DG said.
















Comments
0 comment