views
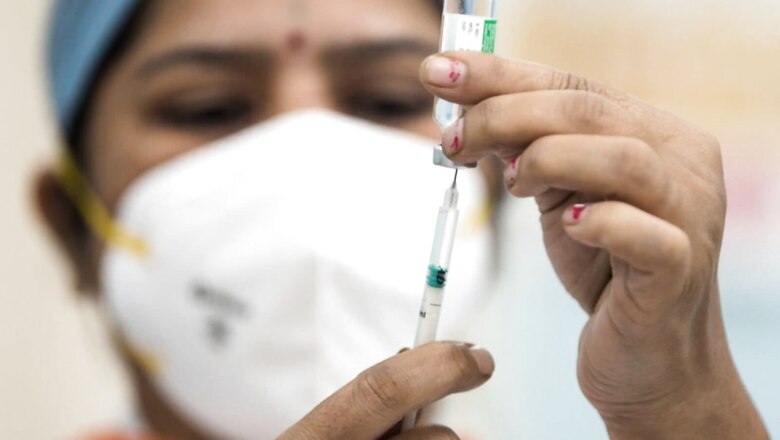
An expert committee will on Monday, February 14, will take a decision on Biological-E’s application to seek nod for use of its Covid-19 vaccine on kids between the age of 12 to 18 years, a report said on the day.
According to a report on the Times of India, a subject expert committee of India’s drug regulator will evaluate Biological E‘s application for approval of its Covid-19 vaccine, Corbevax, on the said group.
Corbevax, if approved, is expected to significantly ramp up the government’s ongoing vaccine drive to vaccinate children between 12 to 15 years of age. This will also be important as schools across the country are opening up amid declining cases of Covid-19.
Biological E had sought for emergency use authorisation of Corbevax for the 12 to 18 years of age group on Sunday, news agency PTI reported. The Drugs Controller General Of India (DCGI) had already given nod to the emergency use of the jab on adults in December last year.
In an application sent to DCGI on February 9, Srinivas Kosaraju, head of Quality and Regulatory Affairs of Biological E Limited, said the firm had received approval for conducting phase 2/3 clinical study of Corbevax among children and adolescents aged 5-18 years in September.
Meanwhile, a News18.com report recently suggested that the Central Drugs Laboratory (CDL), India’s apex lab, has cleared stock of 6 crore Corbevax doses.
The vaccine is currently being stockpiled by the manufacturing firm as the government is yet to decide its usage plan.
A member of the expert panel told News18.com that the use of the new vaccines is only possible as boosters or among children. “There is no need for boosting population right now as a South African study shows Omicron infection induces much higher antibodies or immune response in human body apart from giving protection against Delta variant,” the official said.
Corbevax is India’s first indigenously made RBD protein sub-unit Covid-19 vaccine, develpoed by Hyderabad-based Biological E. According to the Health Ministry, the company has conducted phase 1/2, 2/3 clinical trials of its Covid-19 vaccine in the country. Further, it has conducted a phase 3 active comparator clinical trial to evaluate superiority against Covishield vaccine, it said.
Currently, those above the age of 15 are only eligible for getting vaccinated against Covid-19.
Read all the Latest India News here

















Comments
0 comment