views
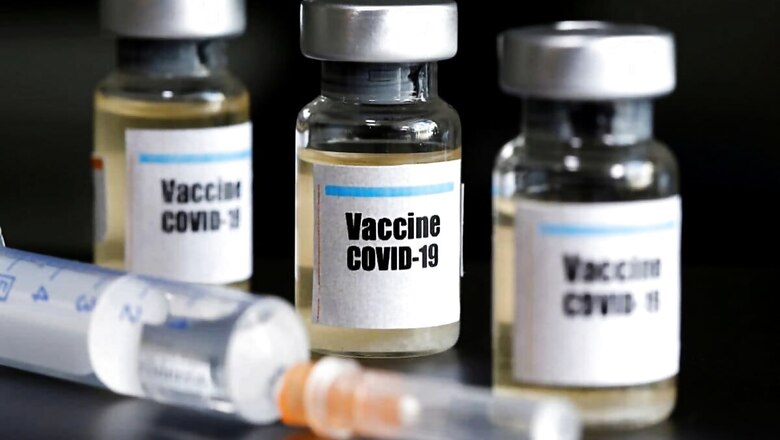
OTTAWA: Canada on Wednesday approved its first COVID-19 vaccine and said initial shots will be delivered and administered across the country starting next week, while every Canadian will be able to be inoculated as early as the end of September.
Canada is the third country after Britain and Bahrain to give the green light to Pfizer Inc’s vaccine, developed with Germany’s BioNTech SE.
Liberal Prime Minister Justin Trudeau said in parliament it was “a good day for Canadians”.
“We will see 30,000 vaccines begin to arrive next week with many more on the horizon, but … we’ve got a tough winter to get through,” Trudeau said.
Canada is in the midst of a virulent second wave of the pandemic, with several areas of the country imposing new health restrictions, weighing down what had been a clear rebound after the first wave.
General inoculations for all Canadians will begin in April after priority populations are taken care of, the government said in a vaccination plan also released on Wednesday.
The nation is now “well-positioned to immunize 100% of the population in 2021,” the plan said, while a separate table distributed by the Health Ministry said immunization could be completed by the end of September. )
“It is going to be good for Canada and the world economy once the vaccinations start rolling out in a meaningful fashion,” said Norman Levine, managing director at Portfolio Management Corp in Toronto.
Toronto’s stock market has rallied about 12% since the end of October and the commodity-linked Canadian dollar has climbed to a 2-1/2-year high against its U.S. counterpart as investors bet that the rollout of vaccines would boost the global economy next year.
The vaccine, which the regulator said was “safe, effective and of good quality,” was approved under an interim, accelerated drug review system very similar to the U.S. Food and Drug Administration’s emergency use authorizations.
It comes a day before a panel of advisers to the FDA is set to review and recommend whether the U.S. agency should authorize use of the Pfizer vaccine.
On Tuesday, British citizens became the first in the world to get the shots outside of clinical trials following last week’s UK approval.
In a large, late-stage trial, the vaccine was shown to be 95% effective at preventing illness, far better than originally anticipated.
However, on Wednesday Britain’s medicine regulator advised that people with a history of significant allergies not get Pfizer’s vaccine after two people reported adverse reactions on the first day of its rollout.
Responding to questions on the allergic reactions, Health Canada officials said that is always a risk with vaccines and that they will monitor developments. They only are recommending that people who have previously had adverse reactions to any of the vaccine’s ingredients not take it for now.
‘HISTORIC MOMENT’
The province of Saskatchewan said on Wednesday it expected to receive enough doses for 1,950 people by Tuesday, intended first for healthcare workers in direct contact with COVID-19 patients.
Quebec expects to have received 55,000 doses of the vaccine by Jan. 4, the premier of the predominantly French-speaking province, Francois Legault, said.
Legault also said he is weighing new restrictions and stepping up enforcement of current ones.
As of Tuesday, Canada had reported 429,035 total cases, an increase of 5,981 on the day before, and 12,867 deaths.
“Today’s decision from Health Canada is a historic moment in our collective fight against the COVID-19 pandemic and is a major step towards returning to normalcy in Canada,” Cole Pinnow, president of Pfizer Canada, said in a statement.
Canada has a firm order for 20 million doses of the vaccine, enough to inoculate 10 million people, with options to buy up to 56 million more. Pfizer will ship its vaccine, which requires ultra-cold storage, to warehouses across Canada.
For the most part, provincial and territorial health systems will administer the shots, for free. They will have final say over how to use scarce early supplies in their jurisdictions.
Priority groups who will get vaccinated first include some healthcare workers, long-term care residents and employees, the elderly and Indigenous populations, according to federal guidelines.
Regulators have received rolling applications for three other experimental vaccines, from Moderna Inc, AstraZeneca Plc and Johnson & Johnson.
The Moderna vaccine is farthest along, having completed pivotal trials showing it to be 94.1% effective at preventing illness and 100% at preventing severe COVID-19.
Officials have said they expect to receive 6 million doses of Pfizer and Moderna vaccines before the end of March. Each vaccine requires two doses given about three weeks apart.
FedEx Corp and Innomar Strategies, a Canada-based unit of U.S. drug distributor AmerisourceBergen Corp, have been hired to provide logistical support on vaccine delivery.
Disclaimer: This post has been auto-published from an agency feed without any modifications to the text and has not been reviewed by an editor
Read all the Latest News, Breaking News and Coronavirus News here













Comments
0 comment