views
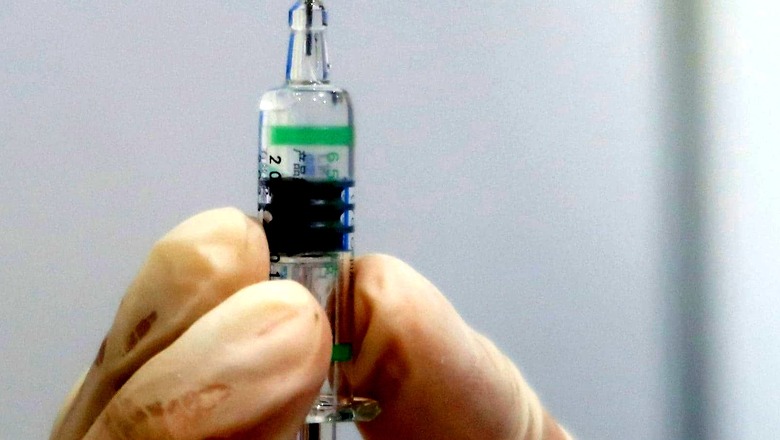
Drug firm Zydus Cadila on Saturday said it is looking to produce 1 crore doses of its Covid-19 vaccine ZyCoVD by October and scale up to 4 to 5 crore doses by end of January. The indigenously developed needle-free three-dose vaccine was granted emergency use authorisation (EUA) by the Drugs Controller General of India on Friday, making it the first vaccine to be administered to beneficiaries in the age group of 12-18 years in the country.
The firm said it is looking to supply its vaccine by the middle to end of September, adding the pricing of the dose will be announced in the next one or two weeks.
“It is a pride moment for us in getting EUA for our first DNA based vaccination. We are glad to work on an indigenous vaccine” Zydus Group Managing Director Sharvil Patel said.
Regarding pricing of the vaccine, the firm said, “Post the emergency use authorisation, now we will work closely with the regulatory authorities to work on the pricing and modality of delivery of the doses of our vaccine. In next one or two weeks we will have better clarity on the pricing.”
Here are the top highlights:
• The company hopes to start supply of the vaccine by middle to end of September.
• Zydus Group Managing Director said, “We believe by October we will start producing 1 crore doses and that would mean by end of Jan we can have 4 to 5 crore doses,” he added.
• On partnering with other firms to scale up production of its vaccine, Patel said Zydus Cadila is actively looking at ramping up manufacturing both domestically through partnerships and also outside of the country.
• The company is also seeking approval for a two-dose regimen of the vaccine, he added.
• It is a needle-free and painless intradermal vaccine.
• Regarding trial results, “None of the subject had any serious severe adverse event. Phase 1 and part of phase 2-3 clinical trial have already been published.” Patel said. Full phase 3 is not fully completed yet so once that is over we will publish that entire data of the vaccine, he added.
• Efficacy of the ZyCoVD three-shots vaccine is 66%.
Apart from adults, adolescents aged 12-18 will take this vaccine. Around 1,000 adolescents were part of the clinical trial.
• The company plans to manufacture 10-12 crore doses of ZyCoV-D annually.
• ZyCoV-D is the sixth vaccine to get the emergency use authorisation in the country, after Serum Institute of India’s Covishield, Bharat Biotech’s Covaxin, Russian vaccine Sputnik V and the vaccines of Moderna and Johnson and Johnson. Of these, Covishield, Covaxin and Sputnik V are currently in use in India. These vaccines are being given to only those above 18 years of age and unlike ZyCoV-D, which has three doses, these are administered in two doses.
“This is a historic milestone with ZyCoV-D, a product of Indian innovation becoming the world’s first DNA vaccine being offered for human use and supporting the world’s largest immunization drive,” Patel had said on Friday.
“We are particularly happy that our vaccine will contribute to this fight against COVID-19 and enable the country to vaccinate a larger population especially in the age group of 12-18 years. “I would like to thank all the researchers, clinical trial investigators, volunteers and the regulators who have supported this endeavour,” the Cadila Healthcare chairman had added.
Describing the approval for Zydus Cadila’s vaccine as a momentous feat, Prime Minister Narendra Modi in a tweet on Friday had said, “India is fighting COVID-19 with full vigour. The approval for world’s first DNA based “ZyCov-D” vaccine of @ZydusUniverse is a testimony to the innovative zeal of India’s scientists. A momentous feat indeed.”
Read all the Latest News, Breaking News and Assembly Elections Live Updates here.















Comments
0 comment