views
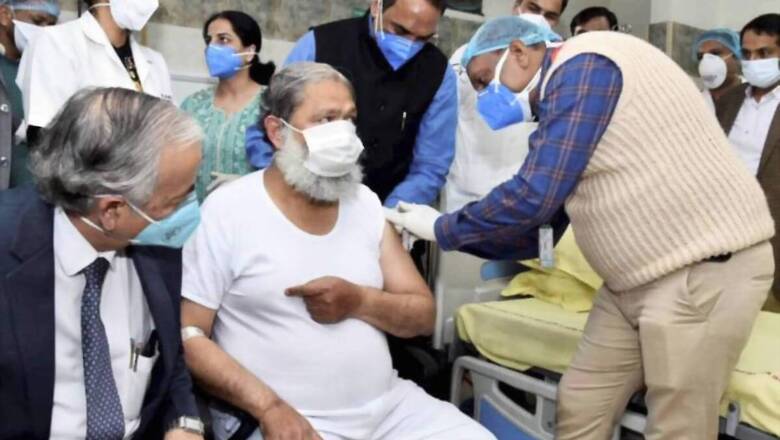
Haryana health minister Anil Vij, who took part in Phase III trials for Bharat Biotech’s coronavirus vaccine Covaxin, has tested positive for Covid-19. “I have been tested Corona positive. I am admitted in Civil Hospital Ambala Cantt. All those who have come in close contact to me are advised to get themselves tested for corona,” Vij tweeted on Sunday.
The development raised concerns over the functioning of trials and efficacy of the vaccine. However, the union health ministry as well as Bharat Biotech were quick to point out that the vaccine’s efficacy would be determined after volunteers receive the full dose.
In the context of the Haryana minister’s tweet, the union health ministry issued a brief comment on the ministry’s official WhatsApp group. “The anti-bodies against the infection build up in a human being only after a specific number of days pass after the second dose of the vaccine is taken. Since this is a two dose vaccine (sic). Minister in question has taken only one dose of the vaccine,” it said.
The union health ministry’s response to Vij’s tweet though raised more questions. The Covaxin Phase III trial has been designed as a randomized, double-blind, placebo-controlled and multi-centre trial to evaluate the safety, immunogenicity and efficacy of the BBV152 vaccine or Covaxin. The Phase III trial will be conducted across 25 centres and 25,800 people are likely to be enrolled.
In a randomised and double-blind trial, neither the volunteer nor the person administering the shot are aware if it is the actual vaccine or the placebo. In the case of Covaxin, it is being compared to phosphate buffered saline with Alum (without antigen). The placebo is to be given in two doses as an intramuscular injection 28 days apart as per the Clinical Trials Registry India (CTRI).
The health ministry’s comment that the “minister in question has taken only one dose of the vaccine”, raises serious ethical issues surrounding the conduct the crucial vaccine trial, which is being carried out in collaboration with Indian Council of Medical Research.
The health ministry’s comment indicates that the trial design has not been complied with. Trials are designed as randomized and double-blind to ensure fairness and rule out any bias in volunteers as well those administering the dose.
News18 reached out to Bharat Biotech seeking a response on the ministry’s comment revealing that the minister received a shot of the vaccine. The company chose not to offer any comment. However, it did issue a general statement which said that the Covaxin clinical trials are based on a two dose schedule, given 28 days apart.
“The vaccine efficacy will be determined 14 days post the 2nd dose. Covaxin has been designed to be efficacious when subjects receive both doses and post the 14 day period after the 2nd dose,” the company’s statement said.
It added, “The phase III trials are double blinded and randomized, where 50% of the subjects will receive vaccine and 50% of subjects will receive placebo.”
The minister’s tweet sent people on social media into tizzy as many perceived it as an indication that the vaccine was ineffective. A volunteer can actually test positive in the middle of the trial as the infection’s viral load reflects through a positive test many days later after the infection.
Vij participated in the trial in a bid to encourage citizens to volunteer in the different clinical trials underway to test vaccines against Covid-19, the disease caused by the novel coronavirus infection. In a much publicised affair, Vij had taken part in the Phase III trials on November 20.
Read all the Latest News, Breaking News and Coronavirus News here

















Comments
0 comment