views
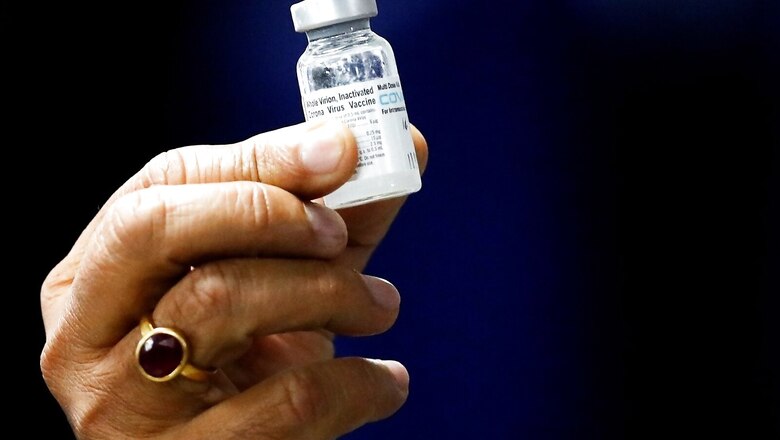
The Drug Controller General of India (DCGI) on Saturday gave Bharat Biotech approval for emergency use authorisation of Covaxin Covid-19 vaccine for children in the 12-18 years age group, according to reports. The Subject Expert Committee (SEC) had recommended DGCI to grant emergency use to Covaxin for children.
Covaxin is now the second vaccine cleared for use for children in India. In August, Zydus Cadila’s three-dose DNA jab was allowed to be used on adults and children over the age of 12. Covaxin will be administered in children in two doses, with a gap of 28 days between the first and second doses. According to the trial data submitted to the government, the gap and dosage of vaccine for adults and children will be the same.
The government, however, has not taken any decision on vaccinating children against COVID-19. The National Expert Group on Vaccine Administration for COVID-19 and National Technical Advisory Group on Immunization are deliberating and considering scientific evidences related to vaccination of those in the 12-17 age group, the Centre recently informed Parliament.
“… Based on the recommendations of SEC experts and submission of additional safety data, this directorate has no objection at this stage for additional indication of Whole Virion inactivated corona virus vaccine for use in age group of more than 12 years to 18 years with the dose schedule of 0 and 28 days for restricted use in emergency situation with the condition to submit SmPC, PI, Factsheet incorporating clinical information for said age group along with pharmacovigilance & risk management plan,” the DCGI approval order issued said.
Bharat Biotech in a statement said, “Covaxin is formulated uniquely such that the same dosage can be administered to adults and children. Covaxin has established a proven record for safety and efficacy in adults for the original variant and subsequent variants. We have documented excellent safety and immunogenicity data readouts in children. We look forward for Covaxin to provide similar levels of protection for adults and children alike.”
Medanta Chairman Dr Naresh Trehan welcomed the announcement and termed it a big relief amid a surge in cases of the Omicron variant of Coronavirus. “In view of Omicron rapidly spreading, it’s great news,” he told News18. “It would help if the data were put out in public,” he said.
Biocon executive chairperson Kiran Mazumdar Shaw called the announcement “extremely important” and said it is a huge relief for parents and schools. “Enough data is showing that children are catching Covid… parents are anxious. Giving vaccines to children will be very useful,” she said.
Bharat Biotech had submitted data from clinical trials in the 2-18 years age group for Covaxin (BBV152) to Central Drugs Standard Control Organisation (CDSCO) in October. The data has been thoroughly reviewed by the CDSCO and SEC and have provided their positive recommendations, the vaccine maker had said in a statement.
Read all the Latest India News here

















Comments
0 comment