views
Understanding Atomic Structures
Begin with the most basic of structures. To pass your chemistry class, you will need to have a good understanding of the building blocks that make up everything that has substance, or mass. The atom is where chemistry begins. Everything in the class will be an extension, built on that basic information. Be sure you take the time to understand the material presented on atoms.
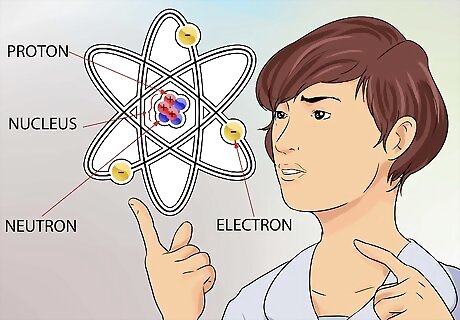
Grasp the concept of an atom. An atom is considered the smallest building block of everything that has mass, including things we can’t always see, like gases. But even the tiny atom has even smaller parts that make up its structure. An atom is made of 3 parts. Those parts are neutrons, protons, and electrons. The center of the atom is called the nucleus. The nucleus is made up of neutrons and protons. Electrons are the particles that float around the exterior part of the atom, like planets circling the sun. The size of an atom is incredibly small. To give some perspective, think about the largest sports arena you know about, maybe the Houston Astrodome. If you consider the Astrodome to be the atom, then the nucleus of that atom is about the size of a pea somewhere around the 50 yard line.
Comprehend the atomic framework of an element. An element is considered to be a substance in nature that cannot be broken down into any other element(s), or to any simpler form. Elements are made of atoms. The atoms of a specific element are always the same. This means that every element has a known, and unique, number of neutrons and protons in its atomic structure.
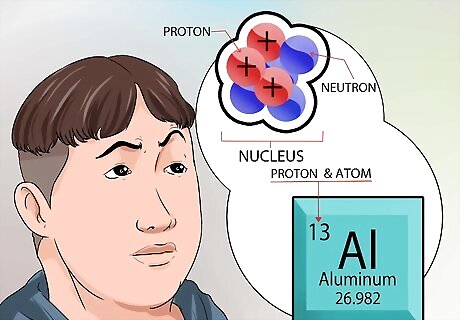
Understand more about the nucleus. The neutrons, found in the nucleus, are neutral in charge. Protons have a positive charge. The atomic number of an element is exactly the same as the number of protons contained in the nucleus. You don’t have to calculate anything to know the number of protons in the nucleus of an element. That number is printed at the top of every squared box, for every element, in the periodic table.
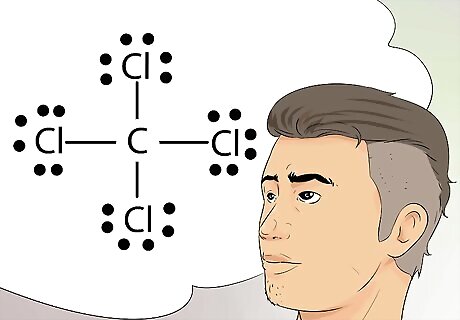
Recognize Lewis diagrams. Lewis diagrams are sometimes called electron-dot diagrams. These are simple diagrams that use dots to represent the paired and unpaired electrons in the outer shell of an atom. Lewis structures are useful in drawing simple diagrams that identify bonds, such as covalent bonds, that are shared between elements in an atom or molecule.
Know what the octet rule means. Lewis diagrams operate on the octet rule, which states that atoms are stable when it has access to eight electrons in the outer shell. Hydrogen is the exception, and is considered stable with two electrons in the outer shell.
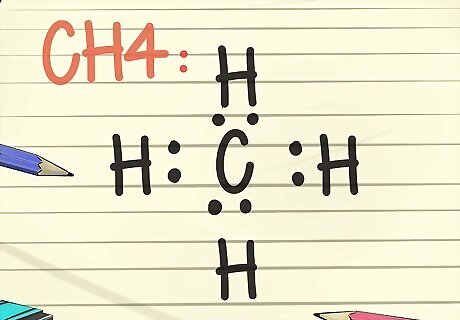
Draw a Lewis diagram. The symbol for an element, surrounded by an arrangement of dots, is a Lewis diagram. Think of the diagram as a still frame of a movie. Instead of the electrons swirling around the outside of the element, they are represented as a fixed moment in time. The diagram shows the stable arrangement of electrons, where they bond to the next element, and information about the strength of the bonds, like if the bonds are shared or doubled. Think about the octet rule, and picture the symbol for an element, perhaps C for carbon. Now place or picture 2 dots at each compass position, meaning 2 dots north of the C, east, west, and south. Now picture an H, representing a hydrogen atom on the other side of each of the 2 dots. This completed Lewis diagram means that the single carbon atom in the center is surrounded by 4 hydrogen atoms. The electrons are bonded in a covalent manner, meaning the carbon and hydrogen atoms share one of their electrons to bond to each other. The molecular formula for this example is CH4, and is the formula for methane gas.
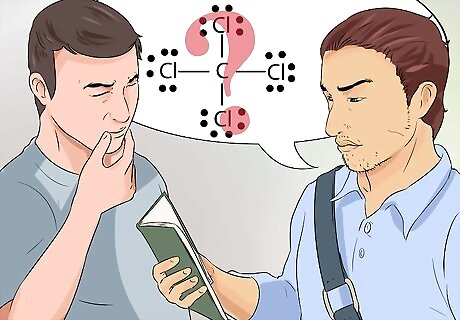
Understand the arrangement of electrons as they bond elements together. The Lewis diagrams are a simplistic visual representation of what is understood about chemical bonds. Talk to your professor, or members of your study group if the concepts about chemical bonding and Lewis diagrams are not clear.
Using the Periodic Table
Look at the periodic table. If you are having trouble with the properties of elements, spend some time reviewing any available material on the periodic table. Most importantly, look closely at one. Understanding the periodic table is critical to passing the first part of your chemistry class.
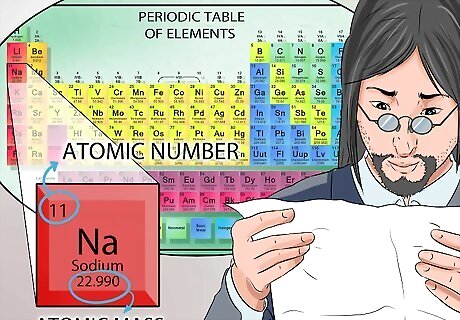
Identify the elements on the periodic table. The periodic table is made up of only elements. Each element has a symbol consisting of either one or two letters. That symbol always identifies that element. Na for example, always means sodium. The complete name of the element appears just below the symbol.
Locate each element’s atomic number. The number above the symbol is the atomic number. The atomic number is the same as the number of protons found in the nucleus.
Find each element’s atomic mass. The number at the bottom is the atomic mass. Remember, the number of protons combined with the number of neutrons found in the nucleus equals the atomic mass number.
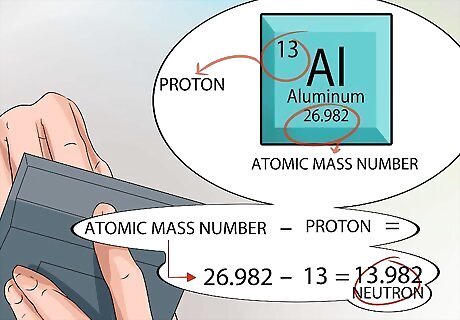
Calculate the number of neutrons found in the nucleus. You can use the numbers provided on the periodic table to figure this out. The atomic number for any element is exactly the same as the number of protons found in the nucleus. The atomic mass unit is printed for every element inside the square at the bottom, just under the name of the element. Remember, the only two things that are in the nucleus of an atom are protons and neutrons. The periodic table tells you the number of protons, and it tells you the atomic mass number. From that point, the math is simple. Subtract the number of protons from the atomic mass number, and that gives you the number of neutrons in the nucleus of every atom for that element.
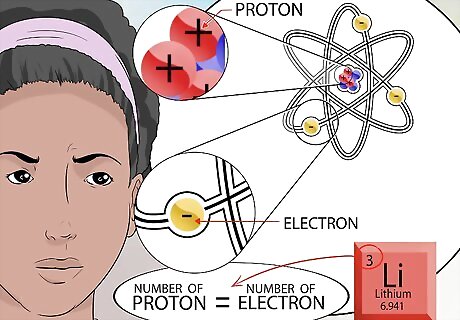
Figure out the number of electrons. Remember that opposites attract. Electrons are positively charged particles that fly around the nucleus of an atom like planets circling the sun. The number of negatively charged electrons that are pulled towards the nucleus, depends on the number of positively charged protons located in the nucleus. Since an atom has no overall charge, all the positive and negative charges contained in the atom must balance. Therefore, the number of electrons is equal to the number of protons.
Predicting Chemical Reactions
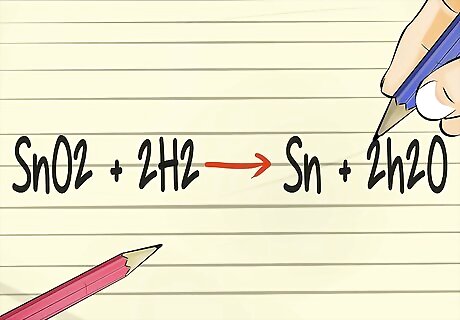
Balance a chemical equation. In a chemistry class, you will be expected to know how to predict what will happen when elements are combined. On paper, this is called balancing chemical equations. The format for a chemical equation consists of reactants on the left side of the equation, then an arrow in the direction of the products of the equation, then the products. The parts on one side of the equation have to balance the parts on the other. For example, Reactant 1 + Reactant 2 → Product 1 + Product 2 Here is an example using the symbols for Tin, which is Sn, in its oxidized form, which is SnO2, combined with hydrogen gas, which is written as H2. SnO2 + H2 → Sn + H2O. But this equation is not balanced since the quantity of reactants must equal the quantity of products. The left side has one more oxygen atom than the right side. Use basic math to balance the equation by indicating 2 hydrogen units on the left side of the equation, and 2 water molecules on the right. The final balanced equation looks like this: SnO2 + 2 H2 → Sn + 2 H2O .
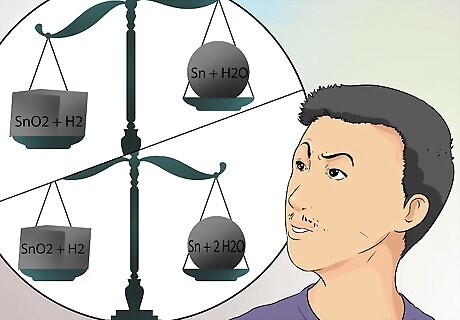
Think about the equations differently. If you are having trouble balancing chemical equations, think about the equation as part of a recipe, but one that needs adjusting on both sides, so you can make more of less of your recipe. The equation gives you the ingredients, on the left side of the equation, but does not tell you how much to use of each ingredient. The equation also tells you what the products will include, but again does not tell you the quantity of the products. You have to figure that out. Using the previous example, SnO2 + H2 → Sn + H2O, consider why this equation, or recipe formula, won’t work. The Sn parts are equal on both sides, and the H2 parts are equal on both sides. But the left side has 2 oxygen parts, and the right side has only 1 oxygen. Change the right side of the equation to indicate the product will contain 2 H2O parts. The 2 in front of the H2O means all the quantities in that grouping are now doubled. So now the oxygen balances, but adding the 2 means there is more hydrogen on the right side of the equation than on the left. Go back to the left and change the H2 ingredient to be twice that, by putting a 2 in front of the H2. Now you have adjusted the ingredients on both sides of the equation. What goes into the recipe and what comes out, are equal, or balanced.
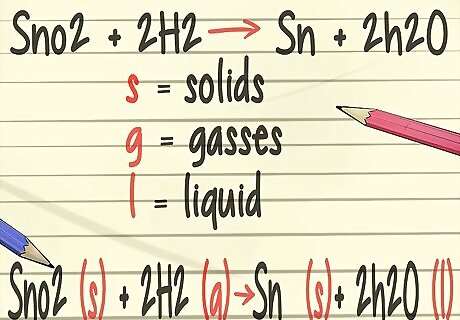
Add more detail to your balanced equations. In your chemistry class, you will learn to add symbols to your balanced equations that represent the physical state of the elements. These symbols will include (s) for solids, (g) for gases, and (l) for liquids.
Identify changes that occur in a chemical reaction. Chemical reactions start with the basic elements, or already combined elements, called reactants. Combining two or more reactants together results in a single product, or several products. To pass chemistry, you will need to know how to solve equations that involve chemical reactants, products, and the introduction of some other influence that alters either the reactants, products, or both.
Identifying Chemical Reactions
Recognize the types of reactions. Chemical reactions can occur as result of many influences, in addition to just simply combining ingredients. Common types of chemical reactions you can expect to learn about include synthesis, analysis, substitution, double displacement, acid-base, oxidation-reduction, combustion, isomerization, and hydrolysis. The types of reactions presented in your chemistry class may vary, depending on the goals of each class. High school chemistry may not provide the same level of detail as that of chemistry taken at a college or university.

Use the resources provided. You will need to grasp the differences in each type of reaction covered in your class. Use resources made available to you by your teacher or professor to understand the different types of reactions covered in your class. Don’t be afraid to ask questions. Understanding the changes that happen with different types of chemical reactions can be confusing. Understanding what happens during specific chemical reactions can be a challenging section of your chemistry class.

Think about chemical reactions logically. Try not to make it harder than it already is by getting caught up in the terminology. The types of chemical reactions you will learn about simply involve doing something to change something. For example, you already know what happens when you combine 2 hydrogen atoms with 1 oxygen atom, you get water. So if you put that water you just made into a pot, and put it on the stove using heat, something changes. You created a chemical reaction. If you put that water into the freezer, same thing. You introduced a change that altered the original reactant, water in this case. Go over each type of reaction one by one until you understand it, then move on to the next type. Focus on the energy source that drives the reaction, and the primary changes that result. If you are having trouble in this area, make a list of what is confusing to you, and go over it with your professor, your study group, or someone that knows chemistry well.
Learning Fluency in Chemistry
Learn how compounds are named. Chemistry has its own rules for nomenclature. The types of reactions that happen to chemical compounds, the loss or gain of electrons in their outer shell, and the stability or instability of compounds are part of the chemistry nomenclature.

Take the section on nomenclature seriously. Most beginning chemistry classes have a section devoted just to nomenclature. In some schools, failure to pass the nomenclature part of the class, means failing the class. If possible, work on nomenclature before you actually begin the class. Many workbooks are available for purchase or through online access.
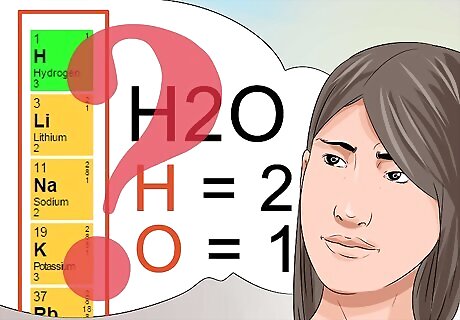
Know what superscript and subscript numbers indicate. Understanding what superscript and subscript numbers mean will be critical to passing your chemistry class. The superscript numbers follow a pattern found in the periodic table, and indicate the overall charge of the element or chemical compound. Review the periodic table to see elements in vertical rows that share the same superscript numbers. Subscript numbers are used to identify the quantity of each identified element that is part of the chemical compound. As previously discussed, the subscript of 2 in the molecule H2O tells you that there are 2 hydrogen atoms as a part of that molecule.

Recognize how atoms react with each other. Part of the nomenclature used in chemistry involves specific rules on naming the products from specific types of reactions. One of those reactions is the oxidative-reduction reaction. This reaction involves the process of either gaining or losing electrons. An easy way to remember the process is to remember the phrase “LEO the lion says GER”. This stands for Lose Electrons in Oxidation, and Gain Electrons in Reduction.
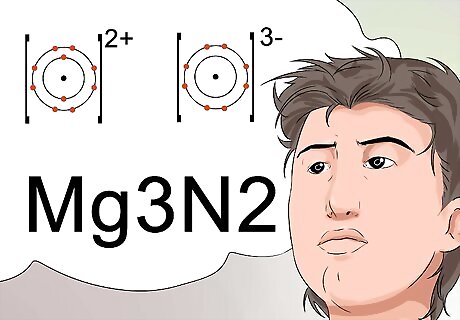
Recognize that subscripts can indicate the formula for a stable charge to a compound. Scientists use subscripts to identify the final molecular formula of a compound, which also indicates a stable compound with a neutral charge. To make a neutral charge, the positively charged ion, called a cation, must be balanced by an equal charge from a negative ion, called an anion. The charges are identified as superscripts. For example, the magnesium ion carries a +2 cation charge, and the nitrogen ion has a -3 anion charge. The +2 and -3 would be indicated as superscripts. To properly combine the two elements to arrive at a neutral charge, 3 magnesium atoms are used for every 2 nitrogen items. The nomenclature that identifies this uses subscripts, and is written as Mg3N2.
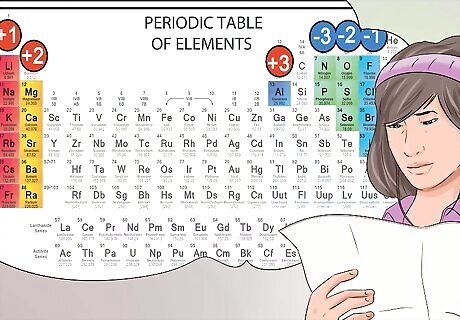
Identify anions and cations from their position on the periodic table. The elements on the periodic table that fall in the first column of elements are considered alkalis, and form +1 cation charges. For example Na+ and Li+. The alkaline earth metals found in the second column form 2+ cations, such as Mg2+ and Ba2+. Elements in the seventh column are called halogens, and form -1 anions, such as Cl- and I-.
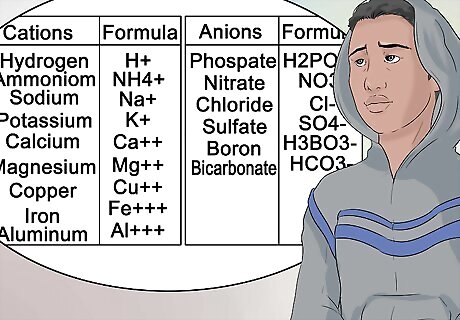
Learn to recognize the more common anions and cations. To help you pass your chemistry class, be as familiar as possible with the nomenclature attached to groups of elements. This type of superscript does not change. In other words, magnesium is always represented as Mg, and always carries a cation charge of +2.
Think of chemistry as learning a new language. Understand that the written forms of indicating charges, the number of atoms in a molecule, and the bonds formed to hold molecules together, are all part of the language of chemistry. All this is a written way of representing what happens in the chemical reactions that can’t actually be seen. It would be so much easier to understand if everything was visible, right in front of you. But in addition to comprehending all the chemistry that is happening, you also have to understand the language used to record and represent everything to do with chemistry. If understanding chemistry is difficult for you, realize that you are not alone, but don’t let it beat you. Talk with your professor, your study group, a teaching assistant, or someone that is really good at chemistry. You can learn all this, but it may help if it can be explained in a way that makes sense to you.
Doing the Math

Know the sequence for basic math calculations. In chemistry, very detailed calculations are sometimes needed, but other times, just basic math skills are adequate. It is important to understand the proper sequence for completing computations in an equation. Memorize a helpful phrase. The phrase, “Please Excuse My Dear Aunt Sally” tells you what applications to perform first. The first letter of each word indicates the order to be used. Anything in Parentheses is done first, then Exponentiation, Multiplication or Division, the lastly Addition or Subtraction. Complete the calculation 3 + 2 x 6 = ___, by ordering your steps according to the phrase. The answer to the equation is 15.

Be comfortable rounding very large numbers. While rounding numbers is not unique to chemistry, the answers to some of the complex math equations result in numbers that are too long to write. Pay close attention to any directions provided in rounding your answers. Know where to round up or down. If the digit next in the series is a 4 or less, then round down, and if it is a 5 or greater round up. For example, consider the number 6.66666666666666. You are asked to round your answer to the second decimal place. The answer is 6.67.
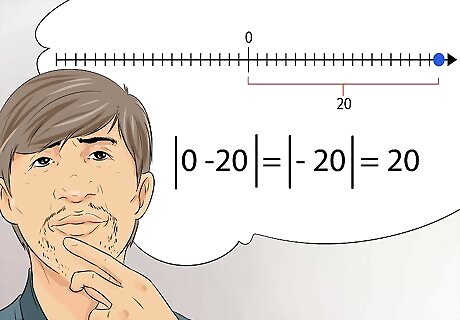
Understand absolute value. In chemistry, some numbers are referred to as absolute value, and not their real mathematical value. Absolute value is the distance from the number to zero. In other words, you no longer consider positive or negatives, just the distance to zero. For example, the absolute value of -20, is 20.
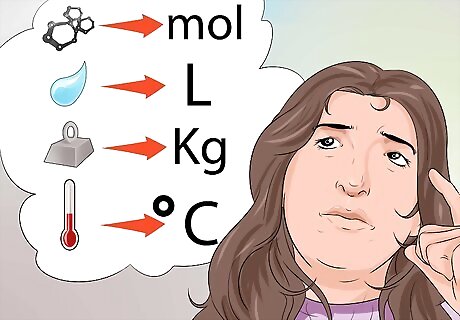
Be familiar with the accepted units of measure. Here are a few examples. Measures of matter are expressed in moles (mol). Temperature is expressed in degrees Fahrenheit (°F), Kelvin (K), or degrees Celsius (°C). Mass is expressed in grams (g), kilograms (kg), or milligrams (mg). Liquid measures are expressed in liters (L), or milliliters (mls).

Practice converting from one scale of measure to another. Part of passing your chemistry class will involve making conversions from one accepted scale to another. This may include changing from one temperature measurement to another, changing pounds to kilograms, and ounces to liters. You may be asked to provide answers in units other than what was in the original question. For example, you may be given a temperature equation to solve in Celsius, and asked to give the final answer in Kelvin. Kelvin is the international standard for temperature measurements often used in chemical reactions. Practice changing from degrees Celsius to degrees Kelvin or Fahrenheit.
Take the time to practice. As you are exposed to various conversions in your class, take the time to learn how to convert from one to the other, and back again.
Know how to calculate concentrations. Sharpen your basic math skills in the areas of percentages, ratios, and proportions.
Practice with nutrition labels on food products. To pass chemistry, you will need to be comfortable calculating ratios, proportions, percentages, and then back again. If this is difficult for you, practice using other common units of measure, like those found on food labels. Look at the nutrition label on any food product. You will see calories per serving, per cent of RDAs, total fat, calories from fat, total carbs, and a breakdown of the different types of carbs. Practice by calculating different ratios and proportions using different categories for the bottom number. For example, calculate the amount of monounsaturated fat per the total amount of fat. Change this to a percent. Calculate how many calories are in the entire container by using the numbers provided for calories per serving and servings per container. Calculate how much sodium is contained in ½ of the full container. By practicing conversions like this, regardless of the units used, you will be much more comfortable exchanging these units of measure for chemistry measures, such as moles per liter, or grams per ml, etc.
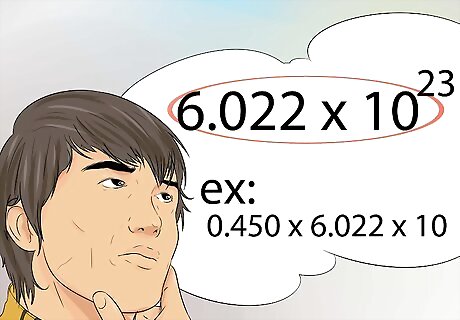
Know how to use Avogadro’s number. This is the number that represents the number of molecules, atoms, or particles, found in one mole. Avogrado’s number is 6.022x10 23. For example, 0.450 moles of Fe contains how many atoms? The answer is 0.450 x 6.022x10 23.
Think about carrots. If you have trouble understanding how to apply Avogadro’s number, think in terms of carrots instead of atoms, molecules, or particles. How many carrots are in a dozen? Well you know that a dozen contains 12 of something, so there are 12 carrots in one dozen. Now answer the question, how many carrots are in a mole? Instead of multiplying by 12, you multiple using Avogadro’s number. So there are 6.022 x 1023 carrots in one mole. Avogadro’s number is used to convert anything of substance, an atom, molecule, particle, or carrot, to how many of that thing is contained in one mole. If you know the number of moles of something, then the final value for the number of molecules, atoms, or particles present, is that number times Avogrado’s number. Understanding how to convert particles to moles is an important part of passing chemistry. Molar conversions are a part of calculating ratios and proportions. This means the quantity of something in moles as a part of something else.
Concentrate on understanding Molarity. Consider the number of moles of something contained in a liquid environment. This example is an important one to understand, since we are now talking about Molarity, or the proportion of something expressed as moles per liter. Molarity is commonly used in chemistry to express the quantity of something in a liquid environment, or the amount of a solute contained in a liquid solution. Molarity is calculated by dividing the moles of solute by the liters of solution. Molarity is expressed as moles per liter. Calculate density. Density is also a commonly used measure in chemistry. Density is the measure of mass per unit volume of a chemical substance. The most common expression for density is given in grams per milliliter, or grams per cubic centimeter, which are the same thing.

Convert equations to their empirical formula. This means that final answers for equations will be considered wrong unless you have broken them down to their simplest form. This does not apply to molecular formulas since that type of description tells you the exact proportions of chemical elements that make up the molecule.
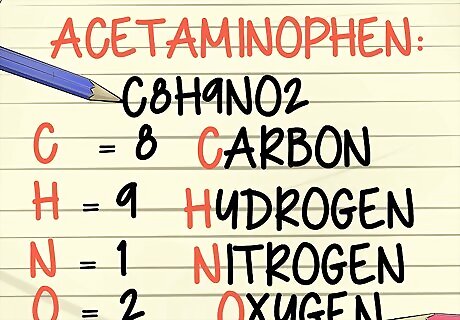
Know what is included in a molecular formula. You don’t change a molecular formula to its simplest, or empirical, form because the molecular formula tells you exactly what makes up the molecule. A molecular formula is written in language that uses the abbreviation of the element(s), and how many atoms of each element, make up the molecule. For example, the molecular formula for water is H2O. This means every water molecule contains 2 hydrogen atoms and 1 oxygen atom. The molecular formula for acetaminophen is C8H9NO2. Every chemical compound is represented by its molecular formula.

Consider chemistry mathematics as stoichiometry. You will likely come across this term. It is a description of the way chemistry is expressed using mathematical formulas. Using chemistry math, or stoichiometry, values of elements and chemical compounds are often represented in terms of moles, molar percent, moles per liter, or moles per kg. As a common math procedure, you will need to convert grams to moles. The atomic mass unit of an element, in grams, is equal to one mole of that substance. For example, calcium has a mass of 40 atomic mass units. Therefore, 40 grams of calcium equals one mole of calcium.
Ask for additional examples. If the math equations and conversions are not coming easily for you, talk to your teacher or professor. Ask for more problems that you can work on your own, until the concepts involved, and all the factors of conversion, make sense to you.
Building Study Habits

Form or join a study group. Do not be embarrassed if chemistry is hard for you. It’s a difficult subject for almost everyone. By working in groups, some members will find areas easier than others, and can help share their methods of learning with the group. Divide and conquer.

Read all of the chapters in your chemistry textbook. Reading a chemistry book is not always the most interesting book on the shelf. But take the time to read the assigned sections, and highlight the parts that just don’t seem to make sense. Try to make a list of questions or concepts that you are having trouble understanding. Go back to those parts later and take a fresh look. If they still seem confusing, talk with your study group, your professor, or a teaching assistant. Try to answer the questions at the end of the chapter. Most textbooks provide additional information that explains the correct answers in case anything is confusing to you. Textbooks use visual aids to get the major teaching points across. Look at the visuals, and pay attention to the captions. This may help to clear some of the confusion.

Ask permission to record the lectures. Taking notes and watching everything the teacher writes on boards or overheads is hard to do, especially in a difficult subject like chemistry. Having a recording that you can listen to again and again may help make it easier for you to understand. However, you should always ask permission to record the lectures before you do so. Try saying something like, “I find it easier to study if I can listen to the lecture over again while I review my notes. Would it be okay if I record your lectures so I can do that?”
Access old tests or study guides. Most natural science courses, such as chemistry, provide access to previous test questions to help students prepare for major tests. Avoid just memorizing the answers. Chemistry is a subject you have to understand in order to answer that same question if it was worded differently.
Getting Help

Get to know your professor or teacher. To pass chemistry with the best grade possible, take the time to meet the person teaching the class. If you are struggling, let them know this is difficult for you. However, even if you are doing well, it is a great idea to get to know the professor. Many professors have study guides available and open additional office hours for student help when needed. Keep a list of areas that are difficult, and ask your professor or teacher for help. This provides you with an opportunity to understand the difficult topics before the class moves on to the next section, and you get even more confused.

Visit online help resources. Pay attention to any online resources or links provided by the chemistry department of your own school.

Try not to become overwhelmed. The detailed information about the different types of chemical reactions, the sharing of electrons, changing the charge of an element or compound, and knowing what the different types of reactions do, can be very confusing. Break down areas that are difficult into describable terms. For example, be able to verbalize that you do not understand oxidation reactions, or how to combine elements with positive and negative charges. By verbalizing the areas you are having difficulty understanding, you may also find some reassurance in realizing there is much you have learned and do understand.

















Comments
0 comment