
views
Preparations

Work in a safe place. This can mean in a clinical laboratory setting. You can find suitable laboratories at most colleges and research institutions. Working at home is also possible but requires caution - manufacturing your own biodiesel may be illegal and can put your house at risk of a fire, smoke or carbon monoxide. A good work place will be well-ventilated and have clear access to running water, eye-wash stations, fire extinguishers, spill containment supplies, a fire alarm and a telephone to use in case of emergencies.

Observe laboratory dress codes. Most laboratories will have posted dress instructions you should follow. You should always wear a long-sleeve shirt, long pants, and shoes in any laboratory setting. When making biodiesel, you should also wear a heavy-duty apron, chemical-resistant gloves (butyl rubber is best when handling methanol and lye) and protective goggles or eyewear. The gloves should come up to your elbows or have cuffs you can pull over your long-sleeve shirt.

Obtain good-quality oil. The easiest oils to use for biodiesel are neutral vegetable oils like canola, corn, and sunflower oil - these oils are readily available at grocery stores and have a low melting point, which means they won't solidify if they get too cold. Avoid using peanut oil, coconut oil, palm oil, tallow, and lard. These sources of oil solidify at relatively high temperatures. Biodiesel usually has a lower melting point than the oil it's made from, but these oils can still be difficult for beginners. Also avoid olive oil. It, peanut oil, palm oil, tallow, and lard all contain more acids than in the recommended neutral oils. These extra acids can interfere with the reactions that take place to create the biodiesel. It's also possible to use waste vegetable oil which has been used for cooking. However, waste oil should be filtered to remove particulates, then allowed to settle for 24 hours to separate the oil from any water or other impurities. Pure oil will be clear and bright, with no sediment.
Ensure all containers are well-labeled. Only use containers for making biodiesel - don't use them for storing food afterwards, even if you wash them well.
Procedure
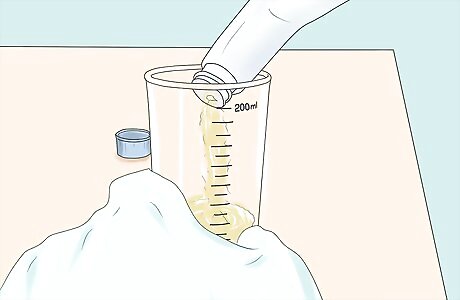
Add 200 ml methanol to glass blender or mixer. Take care not to splash or spill. Set the blender or mixer to "low".

Add 3.5 grams (0.02 oz) of lye. Try to weigh the lye quickly, as it absorbs moisture from the air. For this reason, be sure tightly seal the container you got the lye from. The ensuing reaction between the methanol and lye produces sodium methoxide. Sodium methoxide cannot be allowed to sit for long, as it degrades in the presence of moisture.

Allow the lye to completely dissolve in the methanol. The process should take about two minutes. Proceed when mixture is clear, with no undissolved particles. Again, be attentive - the sodium methoxide will degrade rapidly, so proceed to the next step as soon as the lye is completely dissolved.
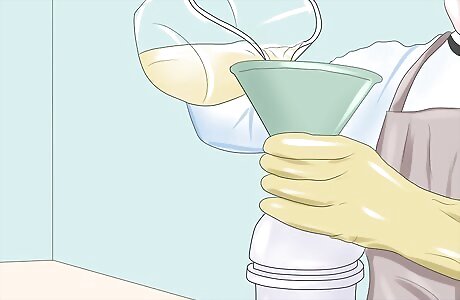
Heat 1 liter (0.3 US gal) of vegetable oil to 130° F (55° C) Add the hot oil to the mixture. Allow the new mixture to blend for about 20-30 minutes. As the reaction proceeds, two products are formed - biodiesel and glycerin.
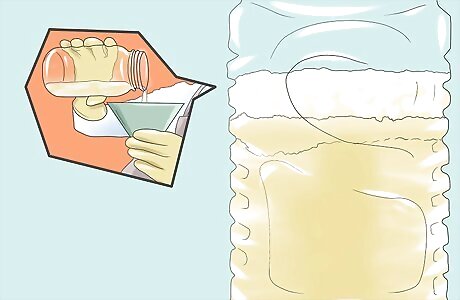
Pour the mixture into a wide-mouthed glass container or pitcher. Allow the mixture to sit. The mixture should separate into two layers - biodiesel and glycerin. Because biodiesel is less dense than glycerin, it should float, forming the top layer.
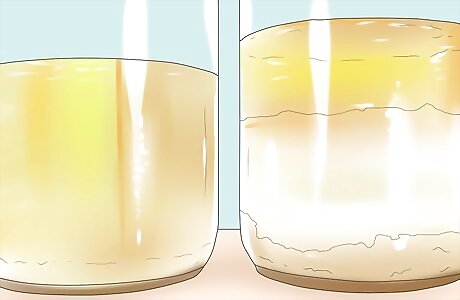
Allow the mixture to sit for several hours. When it has separated completely, carefully keep the top layer to use as your biodiesel fuel. Separate the top layer from the bottom by pouring it off very carefully or using a baster or pump.

Dispose of the glycerin properly. Check with local waste disposal authorities to see whether glycerin can be thrown out with your normal garbage - it usually can. If you don't want to waste your glycerin, consider pouring it on a compost heap to increase the rate of decomposition or using it to make soap. Consult our wikiHow on Making Glycerin Soap for more information.

















Comments
0 comment