views
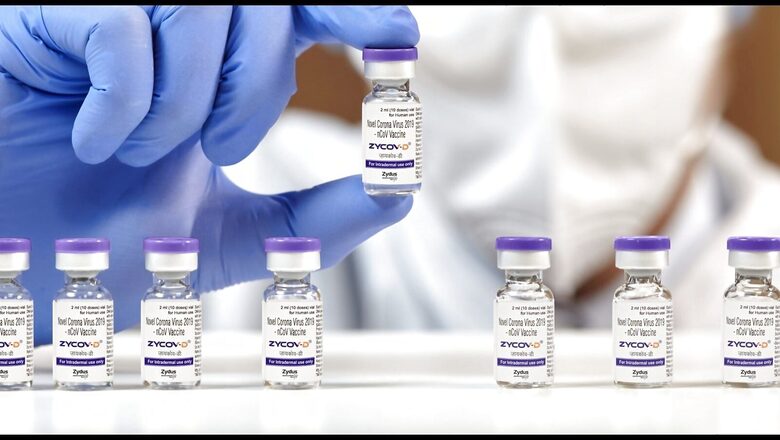
Price of India’s second home-grown Covid-19 vaccine, ZyCoV-D, will be decided basis the technology, capacity and volume, and it will be “affordable”, Dr Sharvil Patel, managing director of Zydus Group, the company manufacturing the vaccine, told News18.com.
ZyCoV-D was approved by the country’s apex drug regulator, Drug Controller General of India (DCGI), on August 20. The first indigenously-developed vaccine in India is Bharat Biotech’s Covaxin.
“The pricing of ZyCoV-D is yet to be finalised in consultation with the government. Technology, capacity and volume will be considered while deciding the pricing,” Patel said, assuring that the final price of vaccine will be “affordable”.
“Throughout the pandemic, Zydus has provided affordable therapeutics, diagnostics and preventives and ZyCoV-D will be no different.”
ZyCoV-D — which is the world’s first plasmid DNA vaccine against Covid-19 — is the only vaccine in India approved for use in the 12-17 years age group, presently.
The company already has stockpiled around 3-5 million doses which can be supplied after they clear the quality and safety checks at the authorised laboratories. “By the end of September, we may see the first dose of vaccines be delivered,” Patel said.
Presently, Zydus Cadila is aiming at a manufacturing capacity of 10-12 crore doses every year.
Planning to seek approval for two dose regimen
Currently a three-dose vaccine — to be administered on day 0, day 28 and day 56 — Patel said that the company is planning to seek approval for the two-dose regimen of the vaccine soon without divulging the exact timeline.
“During the trials of ZyCoV-D, the company had also evaluated a two-dose regimen using a 3 milligram dose per visit and the immunogenicity results were found to be equivalent to the current three dose regimen,” he said.
According to the company, the three-dose vaccine, which is built on the plasmid DNA platform, has 66.6% efficacy against symptomatic Covid-19.
However, the analysis is yet to be peer-reviewed. “While our Phase III clinical trial is still currently underway, we are preparing the manuscript based on the interim analysis and shall be submitting the same for publication to a reputed journal shortly,” he added.
Vaccine stable at 25 degrees for 3 months
Currently, ZyCoV-D is looked at as a potential option for opening schools. On being asked how the company plans to start delivering the vaccine, Patel said that they have completed the logistics planning.
“We are also working on integration with the COWIN app that we will need to do to ensure that we could give better compliance and adherence.”
“We have made sure that we complete all this and I believe that by September we will be well geared to manage the supply chain,” he said while adding that “the fact that ZyCoV-D has demonstrated good stability at 25 degrees for up to 3 months makes logistics and accessibility easier.”
Read all the Latest News, Breaking News and Assembly Elections Live Updates here.


















Comments
0 comment