views
US-based pharmaceutical company Johnson & Johnson said on Monday it was still in talks with the Indian government over its COVID-19 vaccine after the country’s drug regulator announced the company had pulled its proposal seeking an accelerated approval for local trials.
The global healthcare major said it remains committed to bringing its single-dose COVID-19 vaccine to India. “Since the Drugs Controller General of India (DCGI) recently directed that there is no longer a requirement to conduct bridging clinical studies of COVID-19 vaccines in India, Johnson & Johnson withdrew its application to conduct these studies,” the company said.
According to the latest recommendations of the Subject Expert Committee (SEC) meeting to examine COVID-19 related proposals under the accelerated approval process, Johnson & Johnson had informed that it was withdrawing its proposal. “We look forward to ongoing discussions with the Government of India and will continue to explore how to accelerate availability of the Johnson & Johnson COVID-19 vaccine in India,” the company said.
India had, in May, scrapped local trials for “well-established” foreign coronavirus vaccines as it tried to hasten vaccination rollouts to fight a second wave of infections.
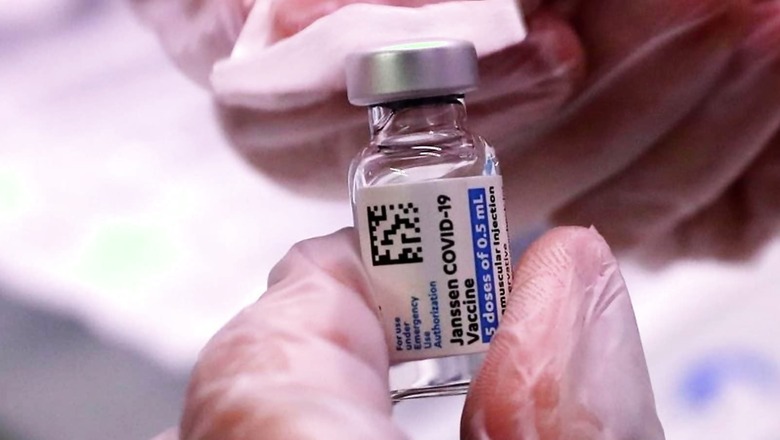
When Did Johnson & Johnson Apply in India?
The Johnson & Johnson had said in April it was seeking an approval to conduct a bridging clinical study of its Janssen COVID-19 vaccine candidate in India. Trials in the United States at that time were paused on reports of rare blood clots and as of July 31, Johnson & Johnson is yet to request a full approval for its shot with the US FDA.
Johnson & Johnson had applied on April 12 in the Global Clinical Trial Division through the Sugam online portal, instead of applying to the biological division which deals with vaccines and other biologicals.
J&J Vaccine’s Safety and Efficacy:
This vaccine has been reviewed by the European Medicines Agency (EMA) and the US Food and Drug Administration (FDA) and found to be safe for people aged 18 and above. Further, clinical trials have found the vaccine efficacious against a variety of SARS-CoV-2 virus variants, including B1.351 (first identified in South Africa) and P.2 (first identified in Brazil).
According to WHO’s interim recommendations for the use of the Janssen Ad26.CoV2.S, the vaccine shows 85.4 per cent efficacy against severe disease and 93.1 per cent against hospitalisation 28 days after inoculation. Clinical trials have shown that a single dose of the J&J vaccine has an efficacy of 66.9 per cent against symptomatic moderate and severe SARS-CoV-2 infection.
The J&J vaccine can be stored for up to three months in a temperature between 2 and 8 degrees Celsius.
Who Can Take the Vaccine?
Persons above the age of 18 can take the J&J vaccine. People with known medical conditions associated with increased risk of severe disease, including chronic lung disease, hypertension, obesity, significant cardiac disease, and diabetes, can be administered the vaccine. Janssen Ad26.COV2.S can also be offered to people who have had COVID-19 in the past. The vaccine has been found to be safe for persons with human immunodeficiency virus (HIV) and can also be offered to a breastfeeding woman. The vaccine can be administered to pregnant women if the benefits of vaccination outweigh the potential risks, according to the WHO’s interim recommendation.
Who Should Not Get Vaccinated?
If you have had a severe allergic reaction (anaphylaxis) or an immediate allergic reaction—even if it was not severe—to any ingredient in the J&J COVID-19 vaccine (such as polysorbate), you should not take the shot. An allergic reaction is considered severe when a person needs to be treated with epinephrine or if they must go to the hospital. Experts refer to severe allergic reactions as anaphylaxis.
People should not take the vaccine if their body temperature is over 38.5ºC. They should postpone vaccination until they no longer have a fever.
Recommended dosage:
According to the Strategic Advisory Group of Experts of WHO, the single dose (0.5 ml) Janssen Ad26.CoV2.S vaccine should be given intramuscularly. Further, there should be an interval of 14 days before the administration of this vaccine and any other vaccine against other health conditions.
Possible Side-effects of the Vaccine:
Pain in the arm, redness or swelling. Tiredness throughout the rest of your body, headache, muscle pain, chills, fever and nausea. These side effects usually start within a day or two of getting the vaccine. Side effects might affect your ability to do daily activities, but they should go away in a few days.
(With inputs from Reuters)
Read all the Latest News, Breaking News and Union Budget 2022 updates here.

















Comments
0 comment